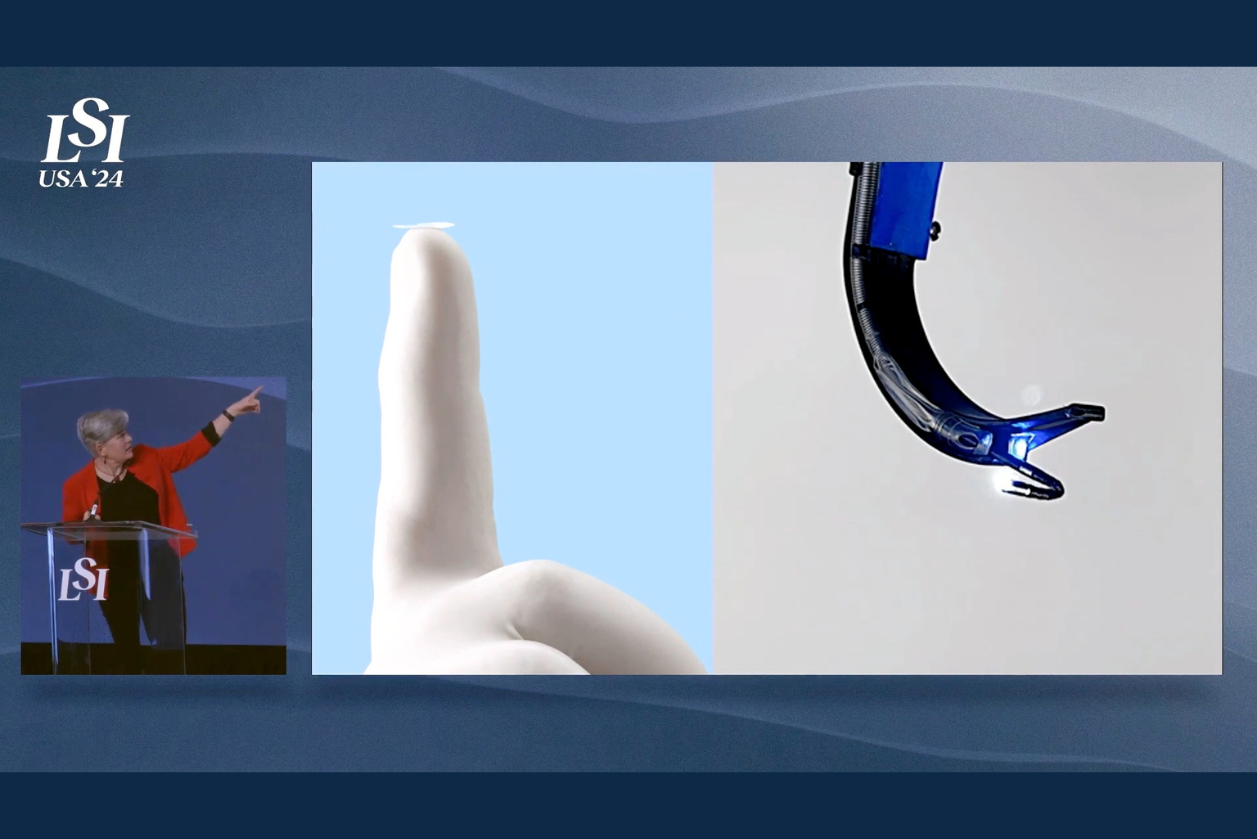
Next-Gen Avisi Technologies Aims for “One and Done” in a MIGS Device
Some medtech start-ups begin with an inventor with a novel idea; others seek out an unmet clinical need looking for a solution. Avisi Technologies began with market research, that is, a direct appeal to glaucoma specialists to determine what they were missing in their tool kits, and specifically, how the current generation of devices for minimally invasive […]